All 11 types of products, including 3 types of permanent products, have completed domestic and international certifications such as KFDA, FDA and started to sell in earnest from May 2021.
ODS started as One Dental System in 2015, changed its name to ODS Co., Ltd. in 2017, and has been steadily focusing on the development of dental resins since then. Currently, ODS commercializes low-viscosity liquid resin and occupies a unique position at home and abroad, and is receiving a lot of attention from dentistry and related industries.
Some resins such as models and casts have been already developed and sold a few years ago, and finally, all 11 types of resins including 3 types of permanent, temporary, denture base, and clear agliner aquiring domestic and international certificates such as KFDA, FDA, and CE, started full-scale sales in May 2021.
ODS, knowing that viscosity is important from the initial development process, focused on realizing the properties of natural teeth at low viscosity.
If the viscosity is high, several properties that can be realized immediately can be achieved quickly, but products with high viscosity have low versatility from the point of view of hospitals or laboratories, such as precision of printing, storability, waste of materials, inconvenience in use, and difficulty in management.
After much trial and error, ODS succeeded in significantly lowering the viscosity to 80 cPs for C&B Permanent A1, A2, A3. Compared to typical materials with a viscosity of 1,000 to 2,000 cPs, this is a groundbreaking achievement.
When the viscosity is low, there is no need to shake or stir for a long time before use, and it is possible to print quickly with a small amount of light, greatly reducing material waste, and greatly improving the convenience of use.
Of course, even if the material of ODS has a low viscosity, it is not lacking in the properties required as a dental material, and is being evaluated by experts as being able to completely replace natural teeth.

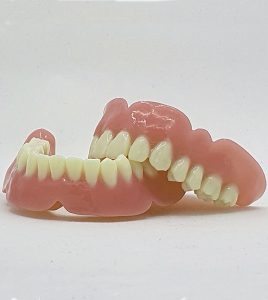
Looking at the currently commercialized ODS materials, they are as follows.
1~3. C&B Permanent A1, A2, A3(Resin for permanent crown & Bridge) / The dental polymer material for producing permanent prostheses such as permanent crowns & bridges manufactured by a 3D printer. – KFDA Certification / FDA, CE in Process
4. CROWN & BRIDGE Temporary(Resin for temporary crown) / The dental polymer material for producing temporary prostheses such as crowns & bridges until the permanent restoration is completed. – KFDA, CE Certification / FDA in Process
5. DENTURE BASE(Light-cured denture base resin) / The resin material for light-curing denture base manufactured using 3D printer for dental only as a dental polymer material for manufacturing denture base. – KFDA Certification / FDA, CE in Process
6. CLEAR(Resin for clear orthodontic appliance) / Resin material for orthodontic appliances manufactured using 3D printers as a dental polymer material used for manufacturing devices used for orthodontic treatment and maintenance. – KFDA Certification / FDA, CE in Process
7. IBT or IDBS(Resin for orthodontic material) / It is called IBT (Indirect Bonding Tray) or IDBS (Indirect Bonding System) for orthodontics, and is a resin material for medical guides used to guide the attachment direction and location of orthodontic bracket. – KFDA Notification / FDA, CE in Process
8. SG(Surgical Guide : Resin for dental implant guide) / Resin material used to create devices used to guide the path, location, and surgical site of implants or instruments using 3D printers. – KFDA Certification, FDA, CE Notification
9. MODEL(Resin for high-precision dental model) / Resin material suitable for high-precision dental model that clearly expresses the fine details of the tooth surface. It can be replaced for all work using existing gypsum models with high strength and low shrinkage and deformation. – No certification required
10. CAST(Ash-Free resin for casting wax) / Ash-free resin material for dental 3D printers with high printing accuracy and speed, no deformation, to completely replacing the existing casting wax for metal casting. – No certification required
11. SCAN CURE(Oral surface recognition improvement material) / During intraoral scanning, Scan Cure is painted to the surface of the area to be scanned, a material that helps oral data to be scanned clearly and quickly. – KFDA Certification / FDA, CE in Process
